UNDERSTANDING YOUR DRUG TARGETS
IN AN EARLY PHASE OF DRUG DEVELOPMENT FOR NEW THERAPEUTICS
TNO combines several of its proven technologies to identify and assess new drug targets, or biomarkers
We can help you to better understand your target in an early phase of drug development. By combining our diverse capabilities, we offer services that extract, integrate and interprets data for your target and translate this into valuable information for decision making.
With a track record in target safety assessment for many years, we do offer additional services in the area of Mechanism Driven Drug Discovery (MD3) and Biomarker Identification.
In addition, we offer tailor-made solutions and have built an AI-based target intelligence platform (TargetTri) to be used as an in-house tool for more efficient target safety assessments, or substantiate early ideation around new drug targets.
TargetTri: Innovating health interventions with evidence-based decision-making
 Powered by custom-tuned large language models, expert knowledge and mined data, TargetTri uncovers crucial insights for assessing health outcomes and safety risks of food and drug interventions.
Powered by custom-tuned large language models, expert knowledge and mined data, TargetTri uncovers crucial insights for assessing health outcomes and safety risks of food and drug interventions.
Query: Drugs, Nutrients or their interacting proteins
Explore: Health, Physiology, Pathology, Disease, Organ
● Evidence-based insights
● Hypothesis generation
● Mechanism of action
● Health benefits
● Safety risks
Efficient Target Profiling and Discovery
Deciding which drug target to push forward in the discovery of innovative therapies requires comprehensive analyses of large amounts of biomedical data, that are scattered and unstructured.
The TargetTri platform tackles this issue with expert knowledge and data-driven approaches, allowing you to spend your time on strategic decision making rather than tedious data collection.
TargetTri provides interactive, target-centric insights in:
● Therapeutic potential
● On-target toxicity
● Drug information
AI-based Target Profiling - TargetTri platform
 Selecting the right target for your next innovative therapy is crucial for the success of the discovery process.
Confident decision making requires the analysis of huge amounts of data from data sources and the literature.
This is time-consuming, repetitive, and prone to user bias. Even worse, details are easily missed in the sea of information.
The TargetTri platform has been developed to overcome these hurdles and ensure you have access to the minute details necessary for accurate decision making.
TargetTri was developed in a close collaboration with the pharmaceutical industry.
It combines custom ontologies, data-mining and data integration with text-mining strategies that allow intuitive inspection of target effect relations on a mechanistic level.
Our text-mining integration allows the exploration of target-related effects from either an organ-centric or effect-centric view. Both diseases and physiology are covered.
Apart from text-mining, TargetTri provides information on target characteristics, diseases, and genetics. Furthermore, it contains compound and drug information for those targets that have advanced in the discovery pipeline.
Our platform particularly focusses on early preclinical development, supporting exploratory target assessments.
It is under continuous further development and we are actively seeking collaborations to tailor the platform to fit your research needs.
Selecting the right target for your next innovative therapy is crucial for the success of the discovery process.
Confident decision making requires the analysis of huge amounts of data from data sources and the literature.
This is time-consuming, repetitive, and prone to user bias. Even worse, details are easily missed in the sea of information.
The TargetTri platform has been developed to overcome these hurdles and ensure you have access to the minute details necessary for accurate decision making.
TargetTri was developed in a close collaboration with the pharmaceutical industry.
It combines custom ontologies, data-mining and data integration with text-mining strategies that allow intuitive inspection of target effect relations on a mechanistic level.
Our text-mining integration allows the exploration of target-related effects from either an organ-centric or effect-centric view. Both diseases and physiology are covered.
Apart from text-mining, TargetTri provides information on target characteristics, diseases, and genetics. Furthermore, it contains compound and drug information for those targets that have advanced in the discovery pipeline.
Our platform particularly focusses on early preclinical development, supporting exploratory target assessments.
It is under continuous further development and we are actively seeking collaborations to tailor the platform to fit your research needs.
Target Safety Assessment(TSA)
TSA combines cheminformatics, network biology, text mining and expert opinion to create a comprehensive report on the expected safety profile of a target.
Such data can then be used in informed decision making around targets, ranking targets, de-risking strategies and/or designing investigative studies.
Our TSA portfolio consist of different products: standard, full or extensive TSAs. Our TSAs are fully modular and can be adjusted to your specific needs.
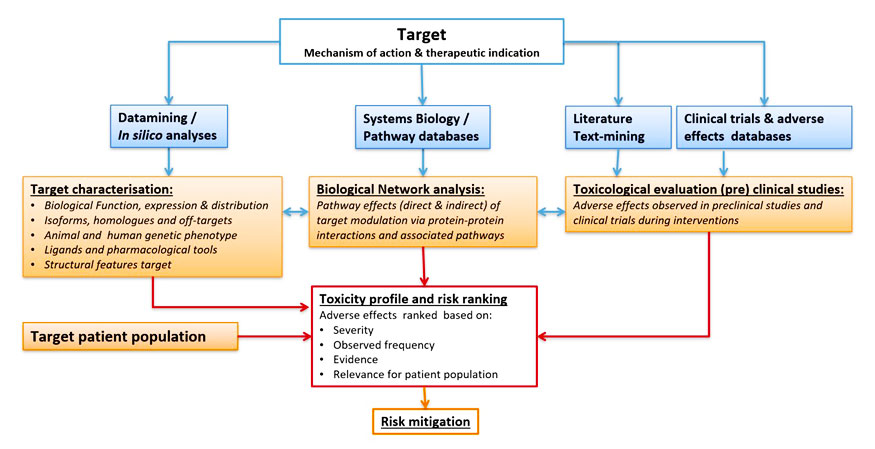
TARGET SAFETY ASSESSMENT(TSA); WORKFLOW
Mechanism Driven Drug Discovery (MD3)
 TNO combines several of its proven technologies(in silico, in vitro, in vivo) to identify new drug targets. Our approach makes it possible:
TNO combines several of its proven technologies(in silico, in vitro, in vivo) to identify new drug targets. Our approach makes it possible:
● to search unexplored areas of biology and select new targets in unknown pathways
● to better understand the molecular mechanism of disease and thereby select safer and more clinically relevant targets
● A first application of MD3 is ongoing for MASH/liver fibrosis, where we have identified 3 targets.
We are looking for pharmaceutical partners to further validate these targets and/or to apply MD3 in other therapeutic domains.
● Publication: Integrating text mining with network models for successful target identification: in vitro validation in MASH-induced liver fibrosis. (Frontiers in Pharmacology, Sep-2024)
The MD3 platform technologies/assets include:
● Well established data- and text-mining technologies (TargetTri)
● Systems biology and AI for the definition of molecular networks related to disease
● Access to and analysis of human samples and preclinical gene expression data sets
● In vitro cell technologies
● Disease knowledge/target evaluation (TSA)
● TNO’s knowledge of preclinical research (obtained in collaborations with Pharma/biotech), we act as a partner
Target Biomarkers Identification (TBI)
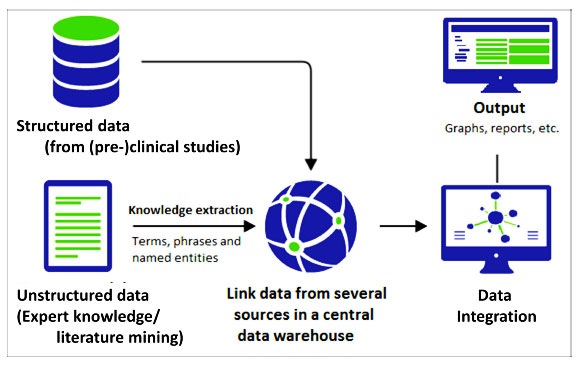 TNO has developed a biomarker workflow to identify biomarkers related to your target of interest. Those biomarkers can be related to safety, efficacy, or other biological phenomena. The identified biomarkers can be monitored during your preclinical and clinical development to assess the target’s effect on safety/efficacy.
The starting point of the Target Biomarker Identification workflow is the drug target that is modulated by the intervention. Applying a systems biology integration approach that mainly uses database information results in candidate biomarkers, which we will prioritize based on additional data (for example if the biomarker can be measured in plasma). The Biomarker Identification assessment can either be based on in silico prediction (on publicly available data) or on data derived in your (pre)clinical studies.
TNO has developed a biomarker workflow to identify biomarkers related to your target of interest. Those biomarkers can be related to safety, efficacy, or other biological phenomena. The identified biomarkers can be monitored during your preclinical and clinical development to assess the target’s effect on safety/efficacy.
The starting point of the Target Biomarker Identification workflow is the drug target that is modulated by the intervention. Applying a systems biology integration approach that mainly uses database information results in candidate biomarkers, which we will prioritize based on additional data (for example if the biomarker can be measured in plasma). The Biomarker Identification assessment can either be based on in silico prediction (on publicly available data) or on data derived in your (pre)clinical studies.
Technical info download
● Publication: Integrating text mining with network models for successful target identification: in vitro validation in MASH-induced liver fibrosis. (Frontiers in Pharmacology, Sep-2024)
This paper describes TNOs integrated target discovery approach, leveraging our TargetTri text-mining, Target evaluation and biological network modeling capabilities in combination with in silico knockout studies. Using metabolic dysfunction-associated steatohepatitis (MASH)-induced liver fibrosis as example, proof-of-concept of was reached by the identification and in vitro validation of three out of five top-ranked candidate drug targets.
The validated in silico pipeline presents a unique approach for the identification of human-disease-mechanism-relevant drug targets. By including directionality in the network, adherence to physiologically relevant signaling cascades is ensured. At the same time, the inclusion of clinical data boosts its translational power and ensures identification of the most relevant disease pathways. Our pipeline thus provide crucial molecular insights for successful target identification.
● Target Safety Assessment (Brochure)
● TargetTri: Safety Assessment and De-risking of Novel Drug Targets, (Poster at ACT 2019)
● TargetTri: A Toxicity Based Triaging System for Novel Drug Targets (Poster at SPS 2017)
● Holistic Approach to the Safety Assessment of Exploratory Drug Targets (Poster at EuroTox 2015)
● Identification and Verification of Functional Biomarkers for Early Detection of NASH-induced Fibrosis.(Poster at Keystone 2019)
● A Discovery and Evaluation Workflow to Identify Novel Targets for NASH-induced Liver Fibrosis (Poster at Discovery on Target 2019)

