HUMAN MODEL TO STUDY INTESTINAL ABSORPTION AND GUT WALL PROCESS
AVAILABLE AS CONTRACT SERVICE, ALSO FOR PARTNERING
Ex vivo model; InTESTine (the model with Fresh intestinal tissue segments)
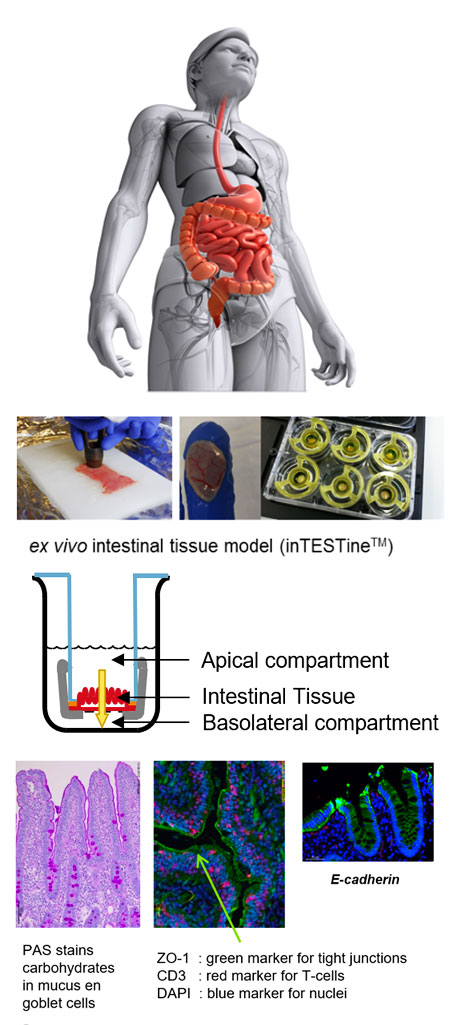
InTESTine is the opportunity to study multiple intestinal segments (duodenum, jejunum, ileum & colon) in parallel, under controlled conditions,
in order to study regional differences in absorption. This is important, as the morphology and function of the intestinal tract changes from duodenum to colon with respect to thickness of the mucus layer, height of the villi, pore size of the tight junctions,
expression levels of transporters, receptors and/or metabolizing enzymes. Additionally, due to the presence of the mucus layer, intestinal processes can be studied following exposure to digested samples, and in the absence, or presence of microbiota.
This clearly demonstrates the additional value of InTESTine compared to monolayer cultures (e.g. Caco-2 cells) in order to study absorption, metabolism and/or food-drug and excipient-drug interactions of orally administered compounds.
Application of fresh intestinal tissue segments.
● Intestinal absorption of nutrients and drugs (small molecure, Peptide, PROTACs, Protein, etc):
● Active transport, metabolism, food-drug effects, excipient-drug effects
● Mucus interactions; Adhesion / Secretion
● Study regional differences Whole GI tract of pigs available
● Effect of compounds on excretion of gut hormones
> Satiety hormones (GLP-1, PYY, GIP, CCK, GLP-2), Serotonin, Melatonin.
● Intestinal Allergen Transport and Basophil activation
● Personal and populational variation in drug absorption via intestinal organoids
● Host-microbe interactions exposure to pathogens
Read-out: Local Immunoresponse (Cytokine, Prostaglandins, etc)
Transcriptomics, Proteomics, Bacterial translation (Pre,Pro-biotics,etc)
Detection methods
● ELISA, Luminex, Fluorescence, FACS, RNAseq, Radiolabels, RT-PCR, qPCR, HPLC/UPLC, LCMS/MS, AMS
Download:
● InTESTine (Brochure)
● Advancing Drug Research with the InTESTineTM Platform: Ex Vivo Intestinal explant Applications for Peptides and Permeability Enhancers. (Poster at the 41th JSSX 2025)
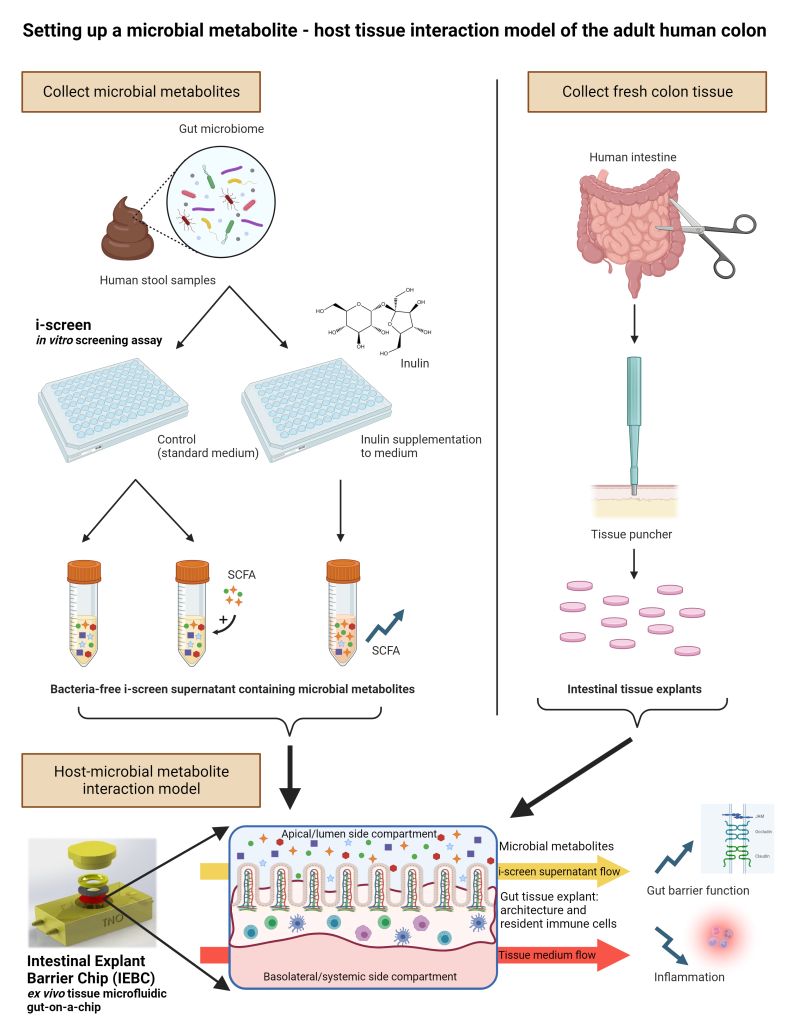
Gut-on-a-Chip, Host-Microbial Interaction model
We aim to develop a physiological in vitro human intestinal model that can be used to study (drug) absorption and impact of drugs, nutrition and microbial
environment on gut health. The ultimate goal is to develop a population-on-a-chip model to aid in the development of precision medicines by targeting patient
variability using intestinal tissue and microbiota from various individuals reflecting populational variation.
Summary:
● A novel microfluidic platform with an enhanced throughput to be able to study (drug) absorption, and impact of drugs, nutrition and microbial environment on gut health.
● Gut-on-a-Chip can be applied to human intestinal tissue biopsies with remained tissue viability and functionality for 26 hours.
● Induction of metabolizing enzymes can be studied.
● We are currently using the Gut-on-a-Chip to study in combination with microbial components to study the influence of host-microbe interactions on drug absorption and immune response
Download:
●
Next Level Drug Research in an Ex vivo tissue Gut-on-a-Chip model: Advanced applications of the intestinal explant barrier chip. (Poster at #38JSSX, 2023)
●
A host-microbial metabolite interaction gut-on-a-chip model of the adult human intestine demonstrates beneficial effects upon inulin treatment of gut microbiome (Microbiome Research Report 2024 )
Publication
Microbiome Research Report 2024
A host-microbial metabolite interaction gut-on-a-chip model of the adult human intestine demonstrates beneficial effects upon inulin treatment of gut microbiome
Joanne M. Donkers, Maria Wiese, Tim J. van den Broek, Esmee Wierenga, Valeria Agamennone, Frank Schuren and Evita van de Steeg.
The Royal Society of Chemistry 2021
Intestinal explant barrier chip: long-term intestinal absorption screening in a novel microphysiological system using tissue explants.
Hossein Eslami Amirabadi, Joanne M. Donkers, Esmee Wierenga, Bastiaan Ingenhut,c Lisanne Pieters, Lianne Stevens, Tim Donkers, Joost Westerhout, Rosalinde Masereeuw, Ivana Bobeldijk-Pastorova, Irene Nooijen and Evita van de Steeg
European Journal of Pharmaceutical Sciences 137 (2019) 104989
A higher throughput and physiologically relevant two-compartmental human ex vivo intestinal tissue system for studying gastrointestinal processes
Lianne J. Stevensa, Marola M.H. van Lipziga, Steven L.A. Erpelincka, Apollo Pronkb, Joost van Gorpb, Heleen M. Wortelboera, Evita van de Steeg.
Beneficial Microbes, 2019; 10(3): 225-236
Mechanisms and immunomodulatory properties of pre- and probiotics
V.B.M. Peters, E. van de Steeg, J. van Bilsen and M. Meijerink
Drug Metabolism and Disposition, Apr, 2017
Regional Expression Levels of Drug Transporters and Metabolizing Enzymes along the Pig and Human Intestinal Tract and Comparison with Caco-2 Cells
Stefan F.C. Vaessen, Marola M.H. van Lipzig, Raymond H.H. Pieters, Cyrille A.M. Krul, Heleen M. Wortelboer, and Evita van de Steeg
Journal of Nutritional Biochemistry 32 (2016) 142-150
Nutrient-induced glucagon like peptide-1 release is modulated by serotonin
Dina Ripkena, Nikkie van der Wielena, Heleen M. Wortelboer, Jocelijn Meijerinkc, Renger F. Witkamp, Henk F.J. Hendriks.
European Journal of Pharmaceutical Sciences 63 (2014) 167-177
A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices
Joost Westerhout, Evita van de Steeg, Dimitri Grossouw, Evelijn E. Zeijdner, Cyrille A.M. Krul, Miriam Verwei, Heleen M. Wortelboer

