in vitro and in vivo models to study mechanisms, interventions and biomarkers
in metabolic diseases and their complications.
Metabolic Health Research (MHR) at TNO
MHR develops and performs in vitro and in vivo models to study mechanisms, interventions and biomarkers in metabolic diseases and their complications.
These translational models include unique (humanized) transgenic mouse models, in vivo and in vitro models, read-out systems employing i.a. histology, biochemical assays, cell biology, molecular biology, immunology and inflammation markers.
This preclinical research is strongly translational and aims to improve the predictability of efficacy and safety of pharmaceutical and food interventions by detailed knowledge of disease processes and mechanisms.
MHR has a track record in applied science, study design, professional project management and quality systems.
MHR offers customized services that can be tailored to customer needs by direct interaction of scientists of MHR and the customer.
We also propose partnering opportunities to gain a deeper understanding of the following points:
● Elucidation of disease mechanisms at the molecular level
● Elucidation of organ cross talk during the disease onset process
● Identification of novel biomarkers
Disease area of Interest
● Obesity, MASLD / MASH
● Sarcopenia / Frailty
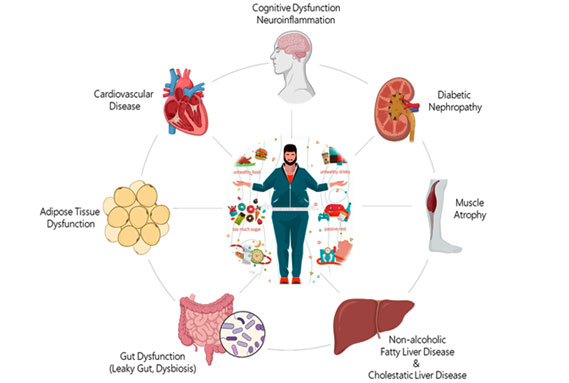
● Cardiovascular-Kidney-Metabolic (CKM) syndrome・Heart Failure(HFpEF)
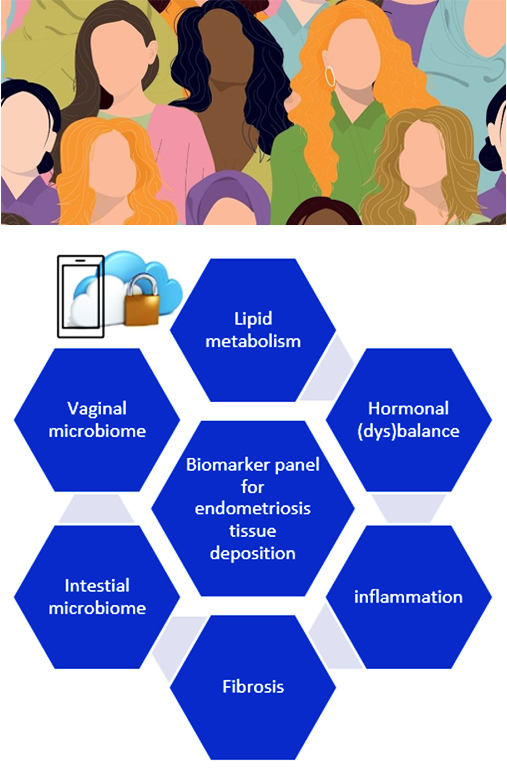
● Woman's Health (Endometoriosis, Menopause, etc)
Women's Health;Health and lifestyle
Our understanding of the female body and its unique healthcare needs remains surprisingly limited. Many drugs are mainly tested on men, and it was only recently that female-specific heart disease symptoms were recognized. Conditions like endometriosis often take an average of 10 years to diagnose. This health disparity also impacts labour productivity, costing billions globally.
TNO's Women's Health program integrates our biomedical, (psycho)social, and technological expertise to tackle women's health challenges. By merging fundamental research with practical solutions, we aim to ensure that future generations of women can achieve their full potential without health-related barriers. However, this goal cannot be reached in isolation.
We collaborate with healthcare professionals, employers, researchers, policymakers, and companies, including those in the food, pharmaceutical and technology sectors.
Current co-development & collaboration opportunities
● Endometriosis: early warning system biomarkers, microbiome and efficacy testing.
● Muscle aging and frailty: cardiac disease, frailty and sarcopenia, optimal performance and sustained employability throughout life.
● Menopause and sex hormones: Key disease drivers and efficacy testing on fibrosis.
● Vaginal health: new in-vitro screenings model.
● Women, work and family health: scan for employers, life cycle perspective on employability, systemic interventions (community approaches, chain approaches), eHealth technologies.
Cardiovascular-Kidney-Metabolic (CKM) syndrome model
 Cardiovascular-Kidney-Metabolic (CKM) Syndrome is a complex disorder involving CVD, kidney disease, type 2 diabetes and obesity.
These conditions share risk factors and can exacerbate each other. Development of new therapies is hampered by the lack of translational models.
A novel diet induced hypertension-accelerated mouse model with obesity, diabetes and hypertension was developed which shows diabetic and chronic kidney disease (DKD/CKD) and heart failure with preserved ejection fraction (HFpEF).
Male KKAy mice on HFD and LNNA developed DKD resulting in CKD and HFpEF.
Combination therapy with Lisinopril and Dapagliflozin rescued GFR decline and reduced renal and cardiac damage.
This indicates the clinical relevance of the model allowing holistic and mechanistic studies in both early and more advanced stages of CKM Syndrome.
Cardiovascular-Kidney-Metabolic (CKM) Syndrome is a complex disorder involving CVD, kidney disease, type 2 diabetes and obesity.
These conditions share risk factors and can exacerbate each other. Development of new therapies is hampered by the lack of translational models.
A novel diet induced hypertension-accelerated mouse model with obesity, diabetes and hypertension was developed which shows diabetic and chronic kidney disease (DKD/CKD) and heart failure with preserved ejection fraction (HFpEF).
Male KKAy mice on HFD and LNNA developed DKD resulting in CKD and HFpEF.
Combination therapy with Lisinopril and Dapagliflozin rescued GFR decline and reduced renal and cardiac damage.
This indicates the clinical relevance of the model allowing holistic and mechanistic studies in both early and more advanced stages of CKM Syndrome.
Features for Cardiovascular-Kidney-Metabolic (CKM) syndrome model
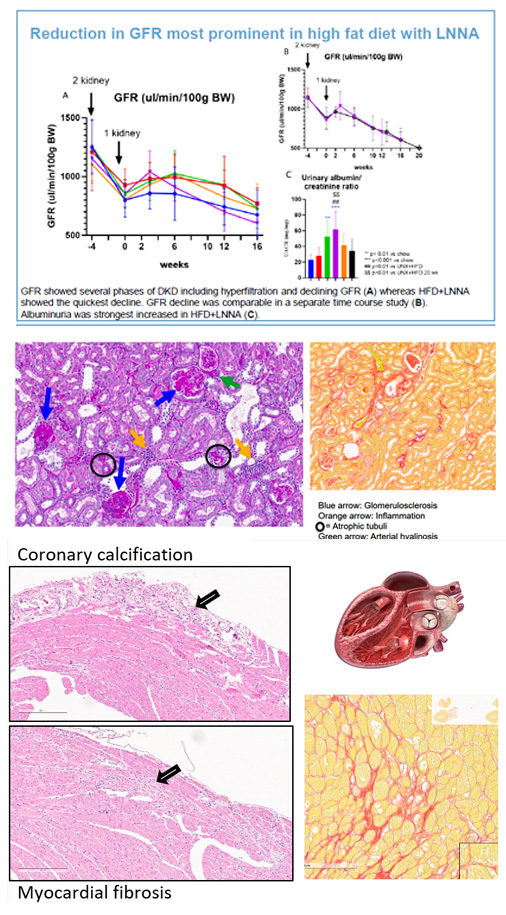
・Progressive decline in renal function in setting of hyperglycemia,
typically preceded by a period of glomerular hyperfiltration.
・Quick onset of Albuminuria
・Pathological changes in kidneys:
> Glomerular basement membrane thickening
> Mesangial matrix expansion and sclerosis
> Tubulo-interstitial fibrosis
> Arteriolar hyalinosis
・Diet induced (Human-like diet composition)
・The model shows hyperlipidemia, hyperglycemia and hypertension, albuminuria, decline in GFR and typical histological features of DKD.
・The model also shows Coronary calcification, Myocardial fibrosis, HFpEF and HFrEF as observed in Heart Failure.
・Reference control: Combination of ACE inhibitor + SGLT2 ihibitor
Readout parameters
・Metabolic parameter: BW, F&W-intake, Glucose, Insulin, CHO, TG
・Function: Diuresis, Albuminuria, UACR, GFR by TD inulin clearance
・Pathology: Quantitative scoring of Glomerular and Tubular damage, Liver and Cardiac damage
・Optional: Proteomics/Metabolomics,Next generation sequencing, EM microscopy, PEMP analysis (NIPOKA)
Download
□
Standard-of-care combination therapy rescues GFR decline and cardiac damage in a novel cardiovascular-kidney-metabolic syndrome mouse model
□
Combination therapy with Lisinopril and Dapagliflozin rescues GFR decline and glomerular damage in the advanced DKD/CKD KKAY mouse model.
□
Cardiac damage in DKD/CKD mouse model resembles HFpEF and can be reduced by Standard-of-care treatment.
Sarcopenia, Frailty models
 The prevalence of sarcopenia is increasing and effective interventions are required to prevent or reverse age-related muscle loss. However, it often is challenging, expensive and time-consuming to develop and test the effectiveness of such interventions and translational animal models that are adequately mimicking the underlying physiological pathways are scarce. Strong predictors for the incidence of sarcopenia include a sedentary life-style and malnutrition.
TNO has therefore recently developed a new and short (2 weeks) mouse model for muscle atrophy that combines caloric restriction with partial immobilization (of one hindleg). This combination model exhibits loss of muscle mass and function. Transcriptome analysis demonstrated that the underlying pathways of this combination model revealed more similarity with the human underlying pathways than aged mice.
In addition to the combination models, we previously demonstrated a beneficial treatment effect in the CR alone model (van den Hoek AM et al., Metabolism 2019).
In addition to the abovementioned models, TNO has several technologies (including AMS; Low dose 14C-alanine incorporation to enable high sensitive detection and tracing of protein turnover in muscle) available for muscle related readouts. In addition, we perform biomarkers research and can support companies with sensors/eHealth solutions in this therapeutic indication.
The prevalence of sarcopenia is increasing and effective interventions are required to prevent or reverse age-related muscle loss. However, it often is challenging, expensive and time-consuming to develop and test the effectiveness of such interventions and translational animal models that are adequately mimicking the underlying physiological pathways are scarce. Strong predictors for the incidence of sarcopenia include a sedentary life-style and malnutrition.
TNO has therefore recently developed a new and short (2 weeks) mouse model for muscle atrophy that combines caloric restriction with partial immobilization (of one hindleg). This combination model exhibits loss of muscle mass and function. Transcriptome analysis demonstrated that the underlying pathways of this combination model revealed more similarity with the human underlying pathways than aged mice.
In addition to the combination models, we previously demonstrated a beneficial treatment effect in the CR alone model (van den Hoek AM et al., Metabolism 2019).
In addition to the abovementioned models, TNO has several technologies (including AMS; Low dose 14C-alanine incorporation to enable high sensitive detection and tracing of protein turnover in muscle) available for muscle related readouts. In addition, we perform biomarkers research and can support companies with sensors/eHealth solutions in this therapeutic indication.
Model option in vitro; in vitro muscle model using C2C12 cells
Readout parameters
・Cell proliferation (number of nuclei per field)
・Differentiation (number of myotubes per field, fusion index, area and diameter of myotubes)
Available technology; EPS (Electrical Pulse Stimulation) to induce contraction
□ Publication:
In vitro muscle contraction: A technical review on electrical pulse stimulation in C2C12 cells, Exp Physiol, Aug 2025
(Watch the video; C2C12 cells that contract 1 second, followed by 4 seconds of rest)
Model options in vivo; C57BL6 mice Regimen; either prophylactic, or therapeutic.
1: Caloric restriction model.
2: Combination model: combining caloric restriction and immobilization.
3: Aged mice.
Readout parameters
・Echo-MRI: Lean body mass/body composition
・Functional tests: Grip strength, inverted screen, voluntary movement/physical activity
・Histology: Cell diameter; slow/fast fiber type, collagen
・Transcriptomics: Pathway analysis, super regulator prediction
・Protein signalling pathways: Pathway analysis (insulin, mTOR, AMPK, FOXO)
・Biochemical: Intramuscular triglyceride content
・AMS: Low dose 14C-alanine incorporation to enable high sensitive detection and tracing of protein turnover in muscle
・Deuterated water can be used for measurement of protein synthesis rate.
□ Recent Publication:
Aged mice model:
Translatability of mouse muscle aging for humans: the role of sex, De Jong et al., Geroscience 2024,
Human study; Sex differences and similarities in muscle aging:
Sex differences in skeletal muscle aging trajectory: same processes, but with a different ranking, De Jong et al., GeroScience 2023
Human study; Sex differences in the development of physical weakness:
Evidence for sex specific intramuscular changes associated to physical weakness in adults older than 75 years, De Jong et al., Biol Sx Diff 2023
Biomarkers for early frailty:
Blood based biomarkers for early frailty are sex specific: validation of a combined in silico prediction and data driven approach, De Jong et al., Geroscience 2024
Rapid Sarcopenia model:
Caloric Restriction Combined with Immobilization as Translational Model for Sarcopenia Expressing Key-Pathways of Human Pathology, Aging and Disease, June 2023
Collaboration program
In addition, TNO is leader of a research consortium that focuses on a better understanding of sarcopenia, including the underlying mechanism.
An unique human cohort is studied to further validate the translatability of our models and to identify biomarkers.
In addition, male/female differences will be studied.
Future directions
・We are also investigating Muscle Health in relation to cognition.
・Muscle health in relation to menopause.
・Muscle Health in relation to the use of GLP-1 analogues (semaglutide/Ozempic).
Fibrosis models
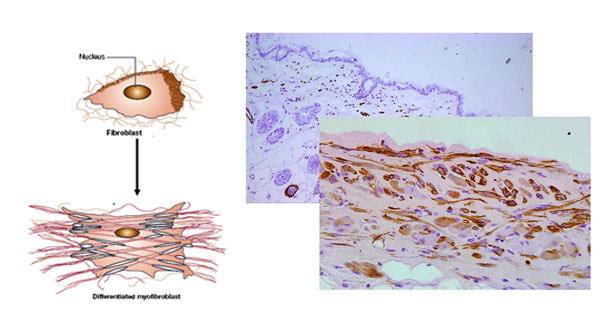
・Lung Bleomycin-induced lung fibrosis model in mice
(Feature:o.p.administration, low variation and motality)
・Skin Bleomycin-induced skin fibrosis model in mice.
・Liver CCL4-induced, Diet-induced model in mice
・Kidney UUO model in mice.
・in vitro fibrosis assay with the fibrosis patient samples
Myoblast differenciation, Fibroblast proliferation, Migration
Current co-development & collaboration opportunities
● Development of Novel Blood-Based Biomarkers for IPF
Recent publication: Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs
We're open to discuss;
・Collagen type analysis:Collagen 1α1, 3α1, 4α1, 5α1, 6α2.
・Signatutre analysis involving newly synthetised collagen
Reference
□ Collagen quantification in cell cultures and tissues.
Cardio Vascular and Metabolic disease models as Familial Hypercholesterolemia and Atherosclerosis
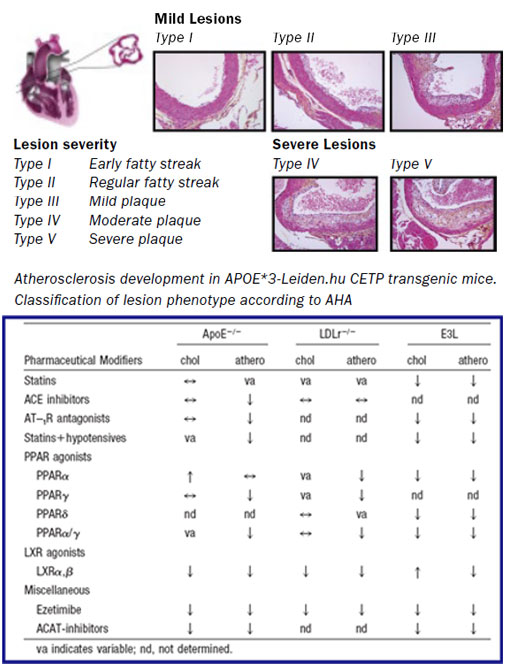 APOE*3 LEIDEN MOUSE
APOE*3 LEIDEN MOUSE
This transgenic mice were generated by the introduction of the human apoe*3-Leiden and apoc1 genes.
The primary effect of the dominant E*3-Leiden mutation is an impaired clearance of triglyceride-rich lipoproteins (chylomicron- and VLDL-remnants) caused by reduced affinity for the LDLR, whereas overexpression of APOC1 inhibits lipolysis.
While normal wild-type mice have a very rapid clearance of apoB containing lipoproteins, E3L mice show an impaired clearance and are thereby mimicking the slow clearance in humans.
As a consequence, E3L mice exhibit a human-like lipoprotein profile comparable to that of patients with familial dysbetalipoproteinemia (most of the circulating cholesterol is contained to (V)LDL particles), and develop atherosclerosis upon feeding with saturated fat and cholesterol. However, E3L mice (like wild-type mice) do not possess a Cholesteryl Ester Transfer Protein (cetp) gene, an essential component of human lipoprotein metabolism, and therefore these mice do not respond to HDL-modulating interventions.
General information
The APOE*3-Leiden.huCETP (E3L.CETP) double transgenic mouse model has proven to be very suitable for testing the effects of drugs and bioactive nutritional components on plasma lipid levels, atherosclerosis, metabolic syndrome and MASH (NASH)-liver fibrosis. The model is based on TNO-Pharma’s proprietary mouse model, the Apolipoprotein E*3-Leiden (APOE*3-Leiden (E3L)) transgenic mouse, an established and well-recognized model for hyperlipidemia and the development of atherosclerosis (reviews Zadelaar et al. ATVB 2007; Kuhnast et al. Eur J Pharmacol 2015; Princen et al. Toxicol Rep 2016) and MASH (a.o. Morrison et al. J Hepatol 2015; van den Hoek et al. Hepatol Comm 2020; van den Hoek et al. Sci Rep 2021). In the E3L.CETP mouse human cholesterol ester transfer protein (huCETP) under control of its natural flanking regions was introduced into the E3L mouse resulting in a more human-like lipoprotein metabolism with transfer of cholesterol ester from HDL to the apoB-containing lipoproteins in exchange for triglycerides. As a result of this adverse lipoprotein distribution and the higher amount of atherogenic apoB-containing (non-HDL) lipoproteins, the E3L.CETP mice develop increased atherosclerosis on a Western-type diet as compared to E3L mice (Westerterp et al. ATVB 2006).
The E3L.CETP and E3L mouse models share the same favorable characteristics, such as (i) responsiveness to all hypolipidemic drugs currently used in the clinic, such as statins, fibrates, niacin, ezetimibe, and PCSK9 mAbs (Kuhnast et al. JLR 2014; Pouwer et al. JLR 2020) and ANGPTL3 mAbs (Dewey et al. NEJM 2017; Pouwer et al. JLR 2020) at similar dosages and in a similar way to humans, (ii) the ability to titrate cholesterol and triglycerides to any desired level and (iii) to conduct atherosclerosis studies in a progression (prevention) design or a regression (therapeutic) design (Pouwer et al. JLR 2020). Moreover, the APOE*3-Leiden.huCETP mouse is very well suited to testing the effect of drugs that modulate HDL and TG levels. The mice demonstrate reduced apoB-containing lipoproteins and increased HDL levels upon treatment with the registered drugs atorvastatin, fenofibrate and niacin, and with CETP-inhibitors (van den Hoek et al. DOM 2014; Kuhnast et al. Eur Heart J 2015).
In contrast to E-/- and LDLR-/- mice the E3L.(CETP) mice possess a functional apoE-LDLR-mediated clearance pathway for non-HDL lipoproteins, providing a suitable model for intervention studies with a.o. PCSK9 and ANGPTL3 inhibitors (Ason et al. JLR 2014; Dewey et al. NEJM 2017; Landlinger et al. Eur Heart J 2017; Suchowerska et al. JLR 2022; Zancanella et al. Mol Therapy Nucl Acids 2023). PCSK9 and ANGPTL3 inhibition with alirocumab or evinacumab demonstrated a significant additional effect on top of a statin with respect to lipid-lowering and atherosclerosis (Kuhnast et al. JLR 2014; Pouwer et al. JLR 2020), resulting in the first study in mice using this combination of clinical hypolipidemic drugs that shows true regression of atherosclerosis (Pouwer et al. JLR 2020).
APOE*3-Leiden/huCETP is a model for mixed dyslipidemia, a condition comparable with diabetic dyslipidemia and it is a predictive animal model: Drugs and nutritional components that failed in clinical studies, such as the GPR109a agonist MK-0354, the CETP-inhibitor torcetrapib (de Haan et al. Circulation 2008), an experimental RCT-inducer, plant sterol derivative and policosanols, also failed in this mouse model. With respect to biologicals, studies have been performed with mAbs, siRNA, miRNA, ASOs, peptides and vaccines against o.a. PCSK9, ANGPTL3, CETP, ox-LDL, Endothelial Lipase, IL-6, and with rec.HDL, GLP-1R, Y2-R and EPO-receptor agonists (a.o. Ason et al. JLR 2014; Kuhnast et al. JLR 2014; Landlinger et al. Eur Heart J 2017; Dewey et al. NEJM 2017; Zancanella et al. Mol Therapy Nucl Acids 2023, Inia et al. JLR 2025).
The mouse models have been used extensively in studies for pharma and food industry in testing efficacy and increasingly safety of small molecules and protein-based therapies and in studies towards the mechanism of action (> 200 studies).
Non-lipid targets and combination studies
The E3L and E3L.CETP mice are well-known mouse models for hyperlipidemia and atherosclerosis with a human-like lipoprotein metabolism which respond to all hypolipidemic drugs currently used in the clinic, such as statins, fibrates, niacin, ezetimibe and PCSK9 and ANGPTL3 inhibitors at similar dosages and in a similar way to humans. However, less well-known, the E3L and E3L.CETP mice are also suitable models for testing the efficacy of anti-inflammatory and blood pressure lowering drugs.
We have published a number of papers which show that modulation of non-lipid targets reduces progression of atherosclerosis, either alone or in combination with a statin, e.g. drugs/compounds with an anti-inflammatory/anti-oxidative action: resolvin E1 (Salic et al. Atherosclerosis 2016); epicatechin (Morrison et al. Atherosclerosis 2014); quercetin (Kleemann et al. Atherosclerosis 2011); salicylate (de Vries-van der Weij et al. Atherosclerosis 2010), all under pro-inflammatory conditions in E3L mice, in absence of cholesterol-lowering effects for resolving E1, epicatechin and quercetin.
And corticosterone (Auvinen et al. Plos One 2013), the SIRT1-activator resveratrol (Berbee et al. J Nutr Biochem 2013) and oncostatin M (van Keulen et al. Plos One 2019) under mild more human-like dietary conditions in E3L.CETP, without affecting plasma lipid levels for corticosterone and oncostatin M.
In addition, anti-hypertensive drugs with different modes of action such as the calcium antagonist amlodipine (Trion et al. J Cardiovasc Pharmacol 2006, efficacy independently of BP-lowering in E3L), the angiotensin II receptor blocker olmesartan (van der Hoorn et al. J Hypertension 2007, in E3L) and the renin inhibitor aliskiren (Kuhnast et al. J Hypertension 2012, in E3L.CETP) also reduce progression of atherosclerosis and improve plaque phenotype without effect on plasma cholesterol levels.
Moreover, the anti-inflammatory effects of a statin have been studied, in which the effects of rosuvastatin were compared to a low-cholesterol control group (matched to achieve the same plasma cholesterol levels of the statin-treated group) to allow investigation of the anti-inflammatory effects independent of the cholesterol-lowering effects of the statin (Kleemann et al. Circulation 2003).
New drugs need to show additional effect on top of a statin, considered to be the golden standard in the clinic. Since the E3L and E3L.(CETP) mice respond to statins as humans do, the model is very suited for combination studies.
Regression of atherosclerosis
With respect to plaque remodeling and regression of atherosclerosis, we recently showed for the first time in mice that true regression of pre-existent atherosclerosis, beyond the lesion size at the start of treatment, is possible in E3L.CETP mice with high-intensive cholesterol-lowering treatment using a combination of three drugs (Pouwer et al. JLR 2020: Alirocumab, evinacumab, and atorvastatin triple therapy regresses plaque lesions and improves lesion composition in mice). Detailed analysis of plaque composition in the latter study, but also from our other studies, indicate that next to regression of lesion size extensive remodeling takes place, with almost complete removal of macrophages and lipids from the plaques and increases in smooth muscle cells and collagen (e.g. Pouwer et al. JLR 2020; Landlinger et al. Eur Heart J 2017; Kuhnast et al. J Hypertension 2012; van der Hoorn et al. Brit J Pharmacol 2009).
Endothelial dysfunction
Together with the Jagiellonian Centre for Experimental Therapeutics at the Jagiellonian University, using sophisticated in vivo measurements of vascular function in the thoracic and abdominal aorta and flow-mediated dilatation in femoral artery using magnetic resonance imaging (MRI), we explored the effects of life-long mild hyperlipidemia on age-dependent endothelial dysfunction (Bar et al. Geroscience 2025). The study showed that endothelial function deteriorates with age, earlier in E3L.CETP male than in female mice. Further, E3L.CETP mice showed faster deterioration compared to wild-type mice, even before atherosclerosis develops. As one cannot study endothelial function and atherosclerosis separately in E-/- or LDLR-/- mice, the E3L.CETP mouse model provides the unique opportunity to study effects of compounds and drugs on endothelial dysfunction induced by dyslipidemia and ageing prior to atherosclerosis development. An example of is given in Kij et al. J Nutr Biochem 2025, showing for the first time that sufficient dietary phylloquinone (a member of the vitamin K family) intake supports endothelial function in normolipidemic and dyslipidemic E3L.CETP mice.
Metabolic syndrome model
In the E3L.CETP mouse on a high fat diet six important parameters of the metabolic syndrome are combined in one animal model which all change in the same direction as observed in humans, i.e. increased body weight and insulin resistance (increased plasma glucose and insulin levels) and at the same time adverse changes in plasma lipids as observed in diabetic dyslipidaemia, with increased triglycerides and apoB-containing lipoproteins and decreased HDL. This mouse model also develops MASLD (formerly known as NAFLD) . The model was validated by intervention with the anti-diabetic drugs rosiglitazone, the GLP-1R agonist liraglutide and an experimental 11β-HSD1-inhibitor, and the lipid-lowering drugs atorvastatin, fenofibrate and niacin, showing simultaneous decreases in plasma triglycerides, cholesterol, glucose and insulin with rosiglitazone, decreases in body weight and plasma glucose and insulin with liraglutide and the 11β-HSD1-inhibitor just as in humans, and decreases in plasma triglycerides and cholesterol with atorvastatin, fenofibrate and niacin, and increased HDL levels with fenofibrate and niacin, also just as in humans. In addition, hepatic triglycerides were significantly decreased by treatment with the high dose of rosiglitazone and liraglutide, while hepatic cholesterol esters were significantly decreased by the high dose of rosiglitazone and atorvastatin (van den Hoek et al. DOM 2014).
Mechanistic studies towards the etiology of high fat-induced insulin resistance and the contribution different organs showed metabolic dysregulation in liver, white adipose tissue and muscle with inflammatory responses in liver and WAT in response to accumulation of lipids (Kleemann et al. PLOS ONE 2010).
MASH-liver fibrosis
When fed a high fat/high cholesterol diet E3L and E3L.CETP mice develop metabolic dysfunction-associated steatohepatitis (MASH) (in about 12-16 weeks) and liver fibrosis (in 20-24 weeks). A general translational MASLD/MASH-liver fibrosis scoring system for rodent models was established based on characteristics in human MASH pathology (Liang et al. PLOS ONE 2014). The model has been validated with clinical candidates as obeticholic acid, elafibranor, icosabutate and several other drug and nutritional interventions (Morrison et al. J Hepatol 2015; Liang et al. Br J Pharmacol 2015; Zimmer et al. Hepatol Comm 2017; van den Hoek et al. Hepatol Comm 2020; van den Hoek et al. Sci Rep 2021; Inia et al. Int J Mol Sci 2023).
An unique feature using this model is the opportunity to conduct intervention studies with two or three relevant clinical endpoints in one animal study, atherosclerosis and MASH-liver fibrosis (Morrison et al. PLOS ONE 2015; Zimmer et al. Hepatol Comm 2017, Inia et al. Int J Mol Sci 2023, and a number of studies underway); and in combination with osteoarthritis (van Gemert et al. Osteoarthritis Cartilage 2021; van Gemert et al. Osteoarthritis Cartilage 2023 (2x)).
Cardiovascular safety
Moreover, due to its sensitivity for modulation of plasma lipids, parameters of the metabolic syndrome, inflammation and atherosclerosis adverse (side-) effects with respect to CV safety can be easily detected (e.g. of the CETP-inhibitor torcetrapib (de Haan et al Circulation 2008), the anti-tumor drug bexarotene (de Vries-van der Weij et al. Endocrinol 2009) and the drugs for chronic myeloid leukemia (CML) imatinib, nilotinib and ponatinib (Pouwer et al. Front Cardiovasc Med 2018; Carracedo et al. Brit J Pharmacol 2022), mineral oil (Pieterman et al. Front Pharmacol 2021), HIV-protease and reverse transcriptase inhibitors, drugs against Gaucher’s Disease, 11β-HSD1-inhibitor, JAK-inhibitors, plant sterol derivative).
With respect to environmental safety and related CV safety issues a series of studies towards the mechanism of action of perfluorosurfactants (resistant to (bio)degradation) on lipid and lipoprotein metabolism were conducted (Bijland et al. Toxicol Sci 2011; Pouwer et al. Toxicol Sci 2019).
Track record
The TNO group has a strong academic background as indicated by the publication list containing many papers on the mechanism of action of drugs, pleiotropic and side effects, often conducted together with partners from industry.
During the past 20 years the department has conducted over 250 projects for industrial partners from pharmaceutical, food and nutrition industry. During the past 3 years on average 15-20 industrial projects per year have been conducted. Client satisfaction score is on average 4.7-4.8 on a scale of 5.
Download
□ Models for Cardiovasucular and Metabolic diseases
□ Cardiovasucular Safety
□ Publication list. ver.Jan-2025
□ Recent Publication:
New Modelity
Proof-of-concept study for liver-directed miQURE technology in a dyslipidemic mouse model. Molecular Therapy Nucleic Acids, June 2023
News March-2025
For over 20 years, TNO’s ApoE*3-Leiden.CETP mouse model has proven to be the most translational model in atherosclerosis research, as evidenced by numerous publications in esteemed, peer-reviewed journals.
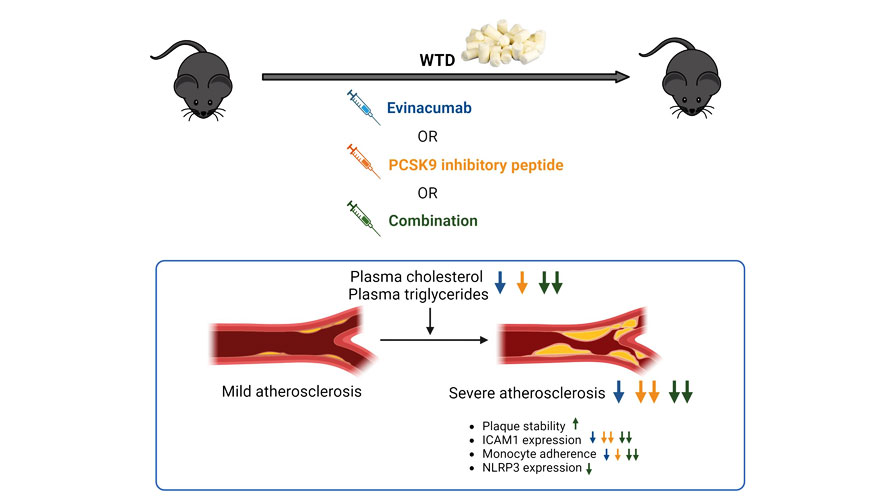
The journey continues! Recently, Jose Inia et al. published a groundbreaking paper titled “Efficacy of a novel PCSK9 inhibitory peptide alone and with evinacumab in a mouse model of atherosclerosis” in the Journal of Lipid Research.
In a previous study, we demonstrated the translational value of the ApoE*3-Leiden.CETP model for evinacumab, highlighting its role in elucidating the underlying processes and mechanisms. The current study’s results suggest that Novo Nordisk’s PCSK9 inhibitory peptide offers a promising approach for patients with atherosclerotic CVD who struggle to meet LDL-C targets with conventional statins or are statin-intolerant.
Read the full paper here: