PRESS and NEWS
-------------------------------
- 2026.1.16 | Clinical Pharmacology | DMPK
Save the date: March 26, 2026; Peregrion B.V. organizes its 2nd AMS symposium.
If your mandate is to develop life changing therapies faster while de-risking development, this year's Peregrion symposium is one you can't afford to miss. For our program we have the privilege to showcase an impressive lineup of international speakers who will share how they leveraged the microtracer AMS approach to tackle diverse ADME challenges and how the microtracer approach helped to accelerate drug development.
Location: Fletcher Wellness-Hotel Leiden
Address:Bargelaan 180, 2333 CW Leiden
Registration: Sign up now

- 2025.11.17 | Translational Research | Metabolic Health
In early November, TNO presented at several major international conferences focusing on obesity, kidney, heart and liver diseases, provided an excellent opportunity for the pharmaceutical and biotechnology industries to discover how TNO's unique strengths in preclinical translational research can support their drug discovery and innovation goals.
Obesity Week in Atlanta, November 4-7 (2 posters)
□ Obesity accelerates age-related memory deficits and affects brain connectivity and white matter tract integrity in Ldlr-/-.Leiden mice.
□ Exercise Partly Protects Against Semaglutide-Induced Muscle Loss in Obese Ldlr-/-.Leiden Mice.
ASN Kidney Week in Houston, November 6-9 (1 oral presentation)
□ Treatment with Either SGLT2 Inhibitors or GLP-1 Receptor Agonists Rescues GFR Decline in a Multifactorial Cardiovascular-Kidney-Metabolic Syndrome Mouse Model.
Scientific Sessions of the American Heart Association in New Orleans, November 7-10 (1 poster)
□ Obicetrapib in combination with ezetimibe on top of atorvastatin regresses atherosclerotic plaque lesions in APOE*3-Leiden.CETP mice.
The Liver Meeting in Washington, November 7-11 (3 posters)
□ Female sex hormones are negatively associated with metabolic dysfunction-associated steatotic liver disease in children with overweight and obesity.
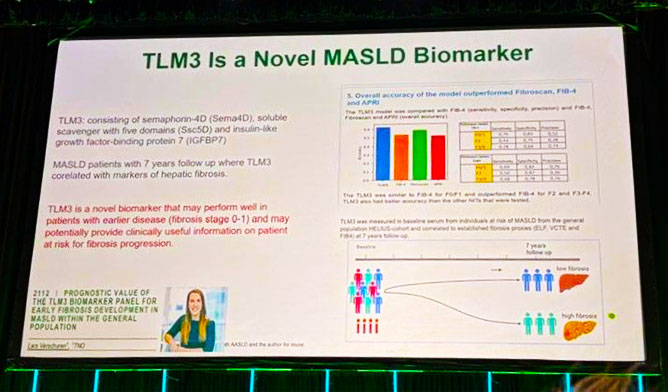
□ Prognostic value of the TLM3 biomarker panel for early fibrosis development in MASLD within the general population.
□ Exercise Partly Protects Against Semaglutide-Induced Muscle Loss in Obese Ldlr-/-.Leiden Mice.
★ Our poster on the TLM3 biomarker for MASLD fibrosis highlighted in the TLM Debrief on Basic Science, Hepatitis, HCC, Cholestatic, MASLD, Alcohol-Associated Liver Disease, and MetALD at AASLD’s Liver Meeting in Washington.
- 2025.10.24 | Clinical Pharmacology | DMPK
TNO presented the following two posters at the 40th Annual Meeting of the Japanese Society for the Study of Xenobiotics held at Miyako Messe in Kyoto from October 20th to 23rd.
□ Normothermic machine perfusion of ex vivo porcine liver: Demonstrator studies for preclinical testing of an RNA therapeutic and multi-day perfusion feasibility. (P-127)
□ Advancing Drug Research with the InTESTineTM Platform: Ex Vivo Intestinal explant Applications for Peptides and Permeability Enhancers. (P-076)

- 2025.10.1 | Clinical Pharmacology | DMPK
A paper has been published in the Antimicrobial Agents and Chemotherapy reporting on a human mass balance study of Acoziborole, a promising single dose oral treatment of human African trypanosomiasis, a deadly disease. TNO, Peregrion (Leiden, the Netherlands) performed the analysis of total radioactivity and the metabolite profiling and identification.
□ Mass balance, pharmacokinetics, metabolism, and excretion of radiolabeled acoziborole, a potential novel treatment for human African trypanosomiasis, following single microtracer oral dose to humans
- 2025.8.20 | Metabolic Health
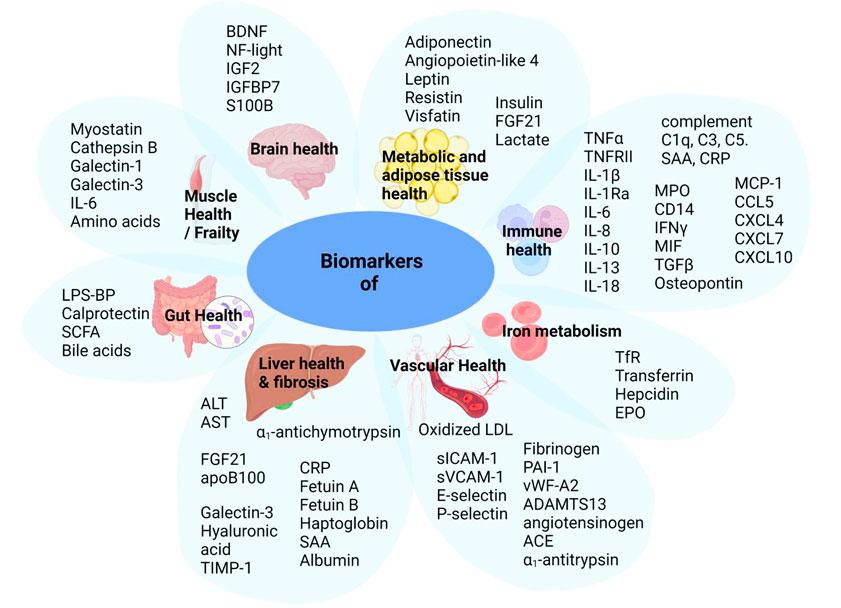
TNO has published a research paper in the International Journal of Molecular Science entitled Biomarkers of Metabolism and Inflammation in Individuals with Obesity and Normal Weight: A Comparative Analysis Exploring Sex Differences. In this study, we analyzed over 90 biomarkers from healthy normal-weight and obese individuals using ELISA and electrochemiluminescence. The results revealed that many biomarkers associated with chronic inflammation and metabolic disorders, such as adipokines and cytokines, were elevated in obese individuals. The study also suggested potential sex differences in biomarker levels in both groups. This dataset not only assists researchers in selecting appropriate biomarkers for their own studies but also highlights the importance of considering sex differences in future research.
- 2025.7.29 | DMPK
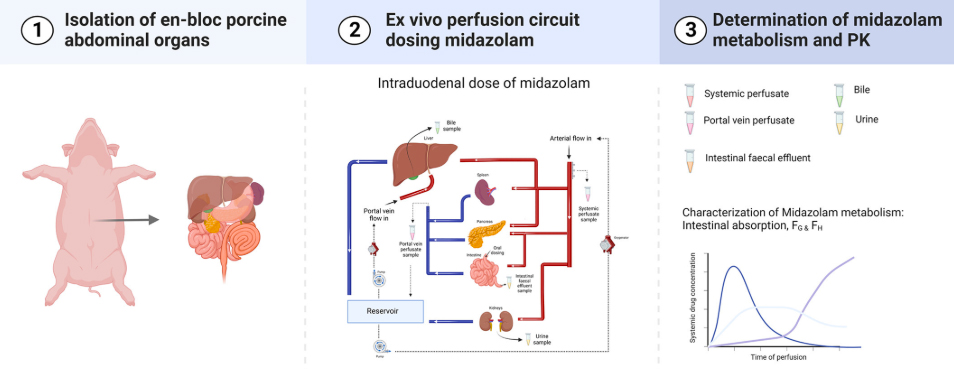
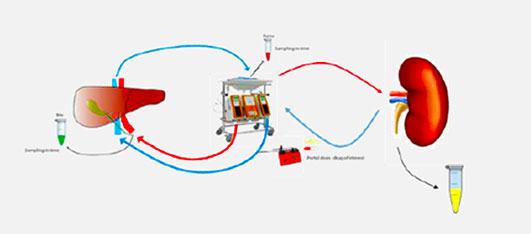
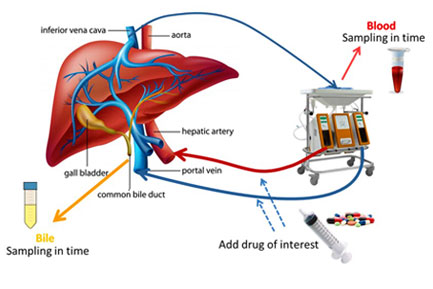
TNO's research on an ex vivo new multi-organ perfusion model that reproduces conditions similar to those in vivo by circulating blood and perfusion fluid through the vascular systems of organs such as the liver, intestines and kidneys, won the Best Paper of the Year Award 2024 from the European Journal of Pharmaceutical Sciences. Link
□ Ex vivo gut-hepato-biliary organ perfusion model to characterize oral absorption, gut-wall metabolism, pre-systemic hepatic metabolism and biliary excretion; application to midazolam
- 2025.5.10 | Metabolic Health
TNO presented the following two research results in poster at EASL congress 2025 held in Amsterdam from May 7th to 10th.
□ Exercise partly protects against semaglutide-induced muscle loss in obese Ldlr-/-.Leiden mice.
□ Anti-inflammatory effects of a chemokine receptor mimicking peptide in obesity-associated MASH and atherosclerosis in Ldlr-/- Leiden mice.

- 2025.3.7| Metabolic Health
TNO scientists participate the 15th International Conference on Frailty and Sarcopenia Research at Toulouse, France from March 12th to 14th, and present the following two research results
□ Exercise protects against semaglutide-induced muscle loss in obese Ldlr-/-.Leiden mice. (March 13, 11:20 AM)
□ Meta-analysis reveals muscle-aging processes are generally shared between females and males at the transcriptome level (Poster)

- 2025.3.6 | Metabolic Health
For over 20 years, TNO’s ApoE*3-Leiden.CETP mouse model has proven to be the most translational model in atherosclerosis research, as evidenced by numerous publications in esteemed, peer-reviewed journals. The journey continues! Recently, Jose Inia et al. published a groundbreaking paper titled “Efficacy of a novel PCSK9 inhibitory peptide alone and with evinacumab in a mouse model of atherosclerosis” in the Journal of Lipid Research. In a previous study, we demonstrated the translational value of the ApoE*3-Leiden.CETP model for evinacumab, highlighting its role in elucidating the underlying processes and mechanisms. The current study’s results suggest that Novo Nordisk’s PCSK9 inhibitory peptide offers a promising approach for patients with atherosclerotic CVD who struggle to meet LDL-C targets with conventional statins or are statin-intolerant. Read the full paper here:

- 2025.2.14 | Metabolic Health
TNO scientists will be participating in Obesity and Adipose Tissue (MASH Pathogenesis and Therapeutic Approaches) to be held at Fairmont Banff Springs, Banff, AB, Canada from February 23rd to 26th, and will present the following two posters.
□ 25 Feb: Resmetirom protects against diet-induced MASLD and reduces atherosclerosis development in obese Ldlr-/-.Leiden mice. (#2519)
□ 26 Feb: TLM3 biomarker panel is prognostic for early MASLD fibrosis development in the general population. (#3052)
- 2025.2.13 | Microbiology, Oral Health
A paper by our Microbiology scientists "Yeast Cell Wall Derivatives as a Potential Strategy for Modulating Oral Microbiota and Dental Plaque Biofilm," has been provisionally accepted by Frontiers. Key Findings: Yeast cell wall derivatives significantly reduced biofilm density and promoted a beneficial shift in microbial composition. Decreased abundance of Tannerella forsythia, a key species in biofilm development. Lowered production of short-chain fatty acids like acetic and butyric acid, positively impacting both healthy and periodontitis microbiomes. These results underline the potential of yeast-based compounds as a sustainable and innovative solution for managing oral health.
□ Yeast cell wall derivatives as a potential strategy for modulating oral microbiota and dental plaque biofilm.
- 2024.11.28 | Metabolic Health
TNO scientists presented the latest findings in MASH research as the followings at AASLD The 75th Liver Meeting in San Diego.
□ Resmetirom protects against diet-induced MASLD and reduces atherosclerosis development in obese Ldlr-/-.Leiden mice.
□ Metabolic flux analysis using a 14C microtracer approach combined with accelerator mass spectrometry analysis - a proof of concept study on de novo lipogenesis.
□ Treatment with the CCR2/CCR5 antagonist Cenicriviroc does not affect MASH and fibrosis development in Ldlr-/-.Leiden mice, translational to clinical phase 3 trial results.
□ Novel blood-based biomarker model for diagnosis of hepatic fibrosis in MASLD.

- 2024.10.30 | General
TNO and the Japanese AIST Group (National Institute of Advanced Industrial Science and Technology (AIST) and AIST Solutions Co (AISol) signed a Memorandum of Understanding (MOU) to expand their existing cooperation across a wide range of technological fields. Related post Link;TNO AIST

- 2024.10.29 | Translational Research
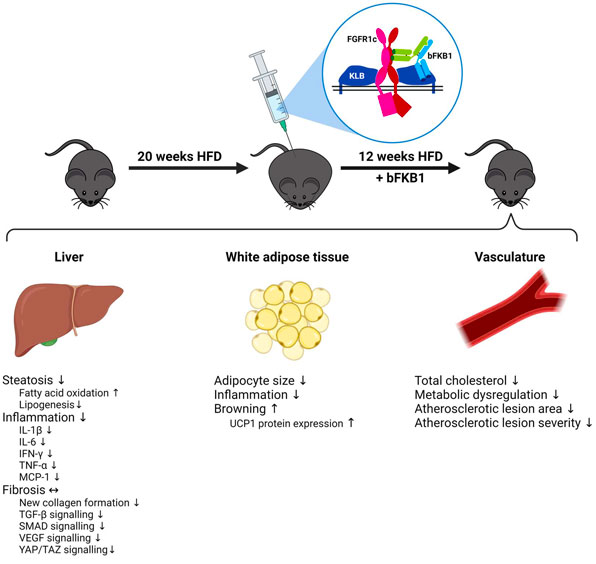
A new paper on the MASH model Ldlr-/-.Leiden mice is published in the FASEB Journal. FGF21 is a promising target for the treatment of obesity-related diseases such as MASH and atherosclerosis, and the pharmacological effect of bispecific anti-FGF21-β klotho (KLB) agonist antibody bFKB1 was evaluated in this model.
□ Therapeutic effects of FGF21 mimetic bFKB1 on MASH and atherosclerosis in Ldlr-/-.Leiden mice
- 2024.7.12 | Translational Research
Another groundbreaking paper on TNO’s biomarkers has been published in a relevant scientific journal: Hogenelst et al. unveiled seven robust and easy-to-obtain biomarkers to measure acute stress in Brain Behavior and Immunity - Health, July 2024. This research focuses on functional biomarkers for stress, surpassing traditional cortisol measurements. It enhances our understanding of stress effects on metabolic health and offers new avenues for stress quantification. These findings are pivotal for stress management interventions, fostering a healthier society. Publication link
- 2024.7.10 | Clinical Pharmacology | DMPK
Peregrion B.V., a 100% subsidiary of TNO specializing in human ADME studies using accelerator mass spectrometry (AMS) and 14C microtracers, has started its contract analysis service as of July 1st. Peregrion B.V. will continue TNO's track record of generating early human data from human ADME studies. TNO has a long history of process optimization, for example developing a fully automated analytical system that can detect, identify and quantify all human metabolites at the early stage of clinical phase I. Currently, the top 20 major pharmaceutical companies are promoting drug development programs using this AMS technology, and industry acceptance is expanding. Press Release link
- 2024.5.29 | Translational Research
Development of a novel non-invasive biomarker panel for hepatic fibrosis in MASLD, which is being promoted by TNO, has been published in Nature Communication
□ Development of a novel non-invasive biomarker panel for hepatic fibrosis in MASLD
- 2024.4.8 | Translational Research
New webpage for in vivo MASH (NASH) model, Liver on a Chip (Cell model for Liver fibrosis) and MASH Biomarkers is introduced in this website. Link
TNO has developed the Ldlr-/-.Leiden MASH mouse model that accurately mimics the etiology and pathology of MASH and fibrosis in humans. By using a high-fat diet, with a macronutrient composition comparable to human diets (e.g., without added cholesterol), the Ldlr-/-.Leiden MASH mouse develops obesity, insulin resistance, adipose tissue inflammation, increased gut permeability with altered microbiota composition, and MASH with bridging fibrosis [F3 stage]. The Ldlr-/-.Leiden MASH mouse has extensive validation records with multiple treatments and drugs, including Semaglutide (GLP-1) and Rezdiffra (Resmetirom) approved by FDA in March 2024.
- 2024.3.22 | Microbiology, DMPK
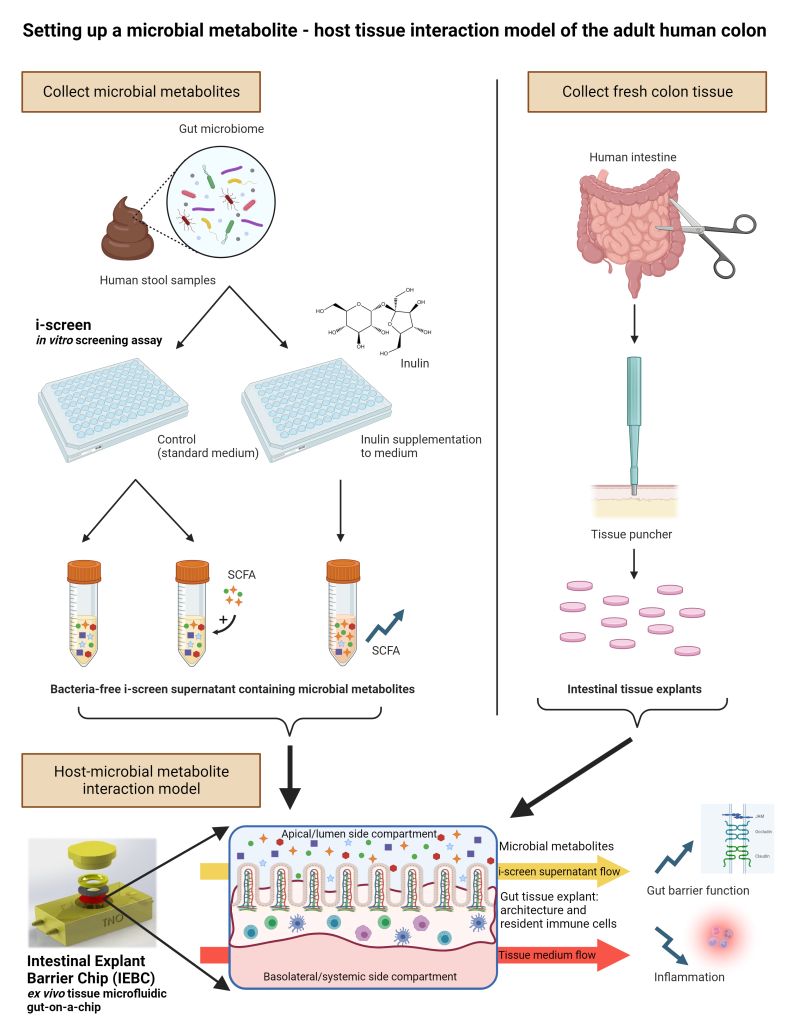
A recent study published in Microbiome Research Report 2024 has demonstrated the beneficial effects of inulin on the gut microbiota using a TNO Gut-on-a-Chip model of the adult human intestine that recapitulates host-microbe metabolite interactions.
The results showed that inulin administration improved the balance of gut bacteria and had a positive impact on health. Publication Link: A host-microbial metabolite interaction gut-on-achip model of the adult human intestine demonstrates beneficial effects upon inulin treatment of gut microbiome
- 2024.3.3 | Metabolic Health
TNO scientists, Reinout Stoop and Arianne van Koppen participate the 6th CKD Summit in Boston on 20 and 21 March.
They will present their poster on TNO's Cardiovascular Kidney Metabolic Syndrome mouse model with CKD and HFpEF.

- 2024.1.12 | Digital HealthTNO joins the iCARE4CVD consortium for personalised cardiovascular care; TNO is one of 33 international partners that have joined forces in the research consortium iCARE4CVD, to better understand cardiovascular disease and optimise future prevention and treatment. By creating one database consisting of data from more than 1 million patients and using artificial intelligence, partners will look for new strategies to shift from a one-size-fits-all approach to personalised care. The consortium brings together international partners from society, academia, and industry, led and coordinated by Maastricht University and Novo Nordisk. Link to Digital Biomarker
- 2023.11.24 | Metabolic HealthTNO presented the latest data of the NASH models at AASLD meeting in Boston, Nov,10-14.
□ Temporal dynamics of metabolic dysfunctions in liver, adipose tissue and the gut during diet-induced NASH development in Ldlr-/-.Leiden mice.
□ Clinical translatability of Ldlr-/-.Leiden mouse model for NASH
- 2023.11.20 | Metabolic HealthTNO presented the latest data of the Diabetic Kidney Disease + HFpEF model at ASN Kidney Week 2023 in Philadelphia, Nov,1-5.
□ Combination therapy with Lisinopril and Dapagliflozin rescues GFR decline and glomerular damage in the advanced DKD/CKD KKAY mouse model.
□ Cardiac damage in DKD/CKD mouse model resembles HFpEF and can be reduced by Standard-of-care treatment.
- 2023.9.29 | DMPK TNO presented the following three posters at 2023 ICCP450/38th JSSX, Shizuoka, Japan, Sep 26-29.
□ Next Level Drug Research in an Ex vivo tissue Gut-on-a-Chip model: Advanced applications of the intestinal explant barrier chip.
□ Optimized Normothermic Machine Perfusion of Liver and Kidney used to Predict Human Pharmacokinetics.
 □
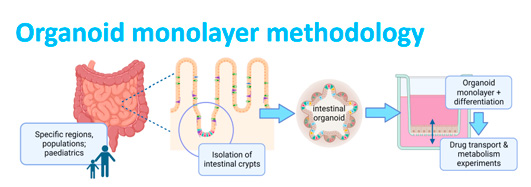
Intestinal Tissue Organoids to study Drug Transport and Metabolism in Various Regions & Age groups.
□
Intestinal Tissue Organoids to study Drug Transport and Metabolism in Various Regions & Age groups.
- 2023.9.1 | Human ADME The publication of the metabolism of LUFOTRELVIR is now available via the following link on Drug Metabolism and Disposition (Sep-2023).
Accelerator Mass Spectrometry was used to generate metabolite profiles of LUFOTRELVIR in human plasma, urine and fecal homogenate.
□ THE METABOLISM OF LUFOTRELVIR, A PRODRUG INVESTIGATED FOR THE TREATMENT OF SARS-COV-2, IN HUMANS FOLLOWING INTRAVENOUS ADMINISTRATION
- 2023.7.26 | Muscle HealthThe new exciting publication on Muscle Health in Biology of Sex Difference. July 2023.
□ Evidence for sex-specific intramuscular changes associated to physical weakness in adults older than 75 years
- 2023.7.25 | New ModelityPOC study with AAV miRNA is published in Molecular Therapy Nucleic Acids, June 2023
□ Proof-of-concept study for liver-directed miQURE technology in a dyslipidemic mouse model
- 2023.5.16 | DMPKA new paper on the ex vivo whole liver organ perfusion model has been published in Clinical Pharmacology and Therapeutics
□ Novel Explanted Human Liver Model to Assess Hepatic Extraction, Biliary Excretion and Transporter Function
- 2023.3.15 | Metabolic HealthTNO scientists (Lars Verschuren, Roeland Hanemaaijer, Martine Morrison and Jose Inia ) participate the Keystone 2023 meeting on Metabolic and Molecular Mechanisms of NAFLD/NASH & Fibrosis Pathogenesis and Resolution:
From Mechanisms to Therapies in Banff, AB, Canada, to be held from 19-23 March. Will present 5 posters entitled as follows:
□ Hepatic molecular signature in a translational NASH model as an early screening tool for novel NASH therapeutics.
□ The bispecific anti-FGFR1/KLB agonist antibody bFKB1 attenuates non-alcoholic steatohepatitis and atherosclerosis in Ldlr-/-.Leiden mice.
□ Semaglutide has beneficial effects on non-alcoholic steatohepatitis in Ldlr-/-.Leiden mice.
□ Translational characterization of the temporal dynamics of metabolic dysfunctions in liver, adipose tissue and the gut during diet-induced NASH development in Ldlr-/-.Leiden mice.
□ Classification (F-score) of NASH-fibrosis patients using blood-based biomarkers related to active extracellular matrix deposition.
□ Early prediction of progressive fibrogenesis in NAFLD-NASH patients using blood-based biomarkers (Oral by Lars Verschuren on 23 March)
All abstracts can be found via the following link: Abstract

- 2023.2.24 | Metabolic HealthTNO scientists (Arianne van Koppen, Reinout Stoop and Robert Ostendorf) will attend the 5th Chronic Kidney Disease Drug Development Summit in Boston on March 8 and 9. We are looking forward to meet the experts in this field to explore options for collaborative programs in gaining more knowledge on the pathway and mechanisms using TNO's model of diabetic kidney disease (DKD). This is an advanced translational diet-induced hypertension-accelerated mouse model of DKD with shows hyperfiltration followed by a steady decline in renal function (GFR), albuminuria, pronounced glomerulosclerosis and tubulo-interstitial atrophy and fibrosis. The model is sensitive to SoC treatment (ACEi and SGLT2i). Additionally, the model shows left ventricle hypertrophy and cardiac fibrosis suggesting HFpEF.
- 2022.11.25 | Analytical Science The following three posters were presented at the European Bioanalytical Forum in Barcelona, Nov, 15-18.
□ De Novo Lipogenesis using 14C-labeled acetate and Accelerator Mass Spectrometry
□ Characterization of hADME and pharmacokinetics of inhaled Velsecorat: alternative use of IV dosing and AMS
□ Accelerator Mass Spectrometry (AMS) Enabled Human ADME study of the FGFR Inhibitor Derazantinib.
- 2022.11.18 | Metabolic HealthThe latest data of the Diabetic Kidney Disease model was presented as a poster at ASN Kidney Week 2022 in Orlando, Nov,3-6.
□ A novel translational model of hypertension-accelerated diet-induced diabetic kidney disease with declining GFR and advanced pathology.
- 2022.10.21 | DMPK TNO presented the following three posters at ISSX MDO2022 in Seattle, September, 11-14.
□ Accelerator Mass Spectrometry (AMS) Enabled Human ADME study of the FGFR Inhibitor Derazantinib.
□ Predicting Human PK by using Ex Vivo Models and PBPK modelling; Demonstrator study using Rosuvastatin and Digoxin.
□ Conversion of Drug Metabolites Back to Parent Drugs by Human Gut Microbiota in an Ex Vivo Fermentation Screening Platform.

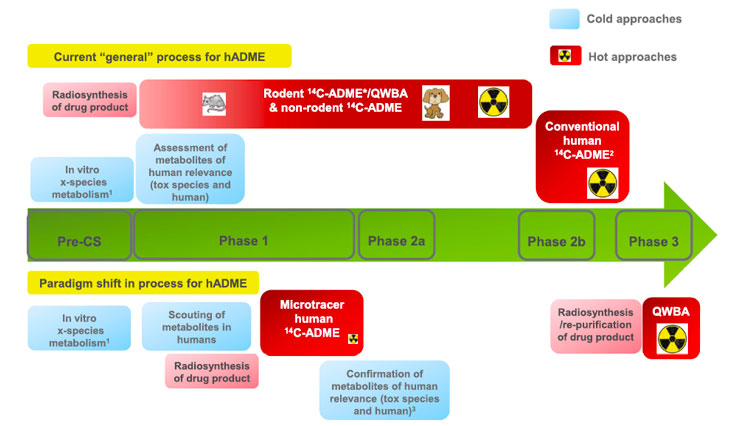
- 2022.9.10 | Clinical Pharmacology | DMPKThe following presentation by Dr. Graeme Young of GSK at the symposium "HUMAN MICROTRACER STUDIES IN DRUG DEVELOPMENT: HOW, WHEN AND WHY" held in Leiden on April 21, 2022 has been published. A must read for all DMPK scientists.
□ Considerations for Human ADME Strategy and Design Paradigm Shift(s)- an Industry White Paper.
- 2022.7.15 | Metabolic HealthIntroduction of a new web-page "Body-Brain Research Interaction Platform".
It is a Research platform for mental health, cognition, stress, performance, wellbeing to comprehensively investigate the efficacy (or adverse effect) of various types of test product or factor such as medicine, nutrient, lifestyle, and psycho-social.
Available for fee for service, Open for partnering depending upon research questions.
□ LINK:BODY BRAIN RESEARCH
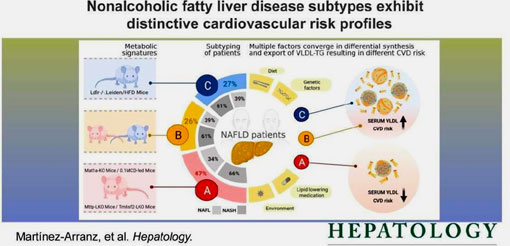
- 2022.6.17 | Metabolic HealthNew publications on the Translationability of LDLr-/-. Leiden mice as NASH, NAFLD model
□ Metabolic subtypes of nonalcoholic fatty liver disease patients exhibit distinctive cardiovascular risk profiles. Hepatology 2022.
 □
The Human Milk Oligosaccharide 2'-Fucosyllactose Alleviates Liver Steatosis, ER Stress and Insulin Resistance by Reducing Hepatic Diacylglycerols and Improved Gut Permeability in Obese LDLr-/-.Leiden Mice.
□
The Human Milk Oligosaccharide 2'-Fucosyllactose Alleviates Liver Steatosis, ER Stress and Insulin Resistance by Reducing Hepatic Diacylglycerols and Improved Gut Permeability in Obese LDLr-/-.Leiden Mice.
- 2022.3.10 | Organ On a ChipOn March 9, TNO's webinar "ORGAN ON A CHIP: BRIDGING BIOLOGY AND TECHNOLOGY IN EFFICACY AND ADME TESTING" took place.
You can watch this webinar on demand via this link:
□ INTRODUCTION TNO AND THE ORGAN ON A CHIP RESEARCH PROGRAMME:
Robert Ostendorf,Ph.D, Senior Business Development Manager, TNO
□ INTESTINE ON A CHIP: IN VITRO ADME MODEL TO STUDY THE EFFECT OF MICROBIOME TOO:
Evita van de Steeg,Ph.D, Senior Scientist, TNO
□ LIVER ON A CHIP - AN IN VITRO EFFICACY MODEL FOR NASH-FIBROSIS
Geurt Stokman,Ph.D, Senior Scientist, TNO
- 2022.2.8 | Clinical PharmacologyICON and TNO cordially invited you to join our Microtracer Symposium on April 21st.
The symposium was held in the Fletcher Hotel Leiden, The Netherlands.
□ Program and Registration: HUMAN MICROTRACER STUDIES IN DRUG DEVELOPMENT: HOW, WHEN AND WHY
How Accelerator Mass Spectrometry accelerates drug development? (Recommended browser: Google Chrome)
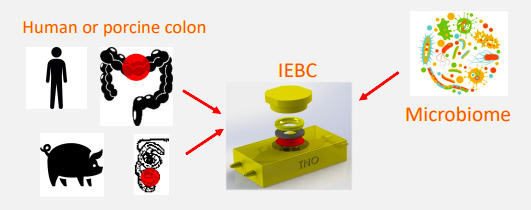
- 2021.12.10 | DMPK Publication on the Intestinal Explant Barrier Chip (IEBC) became available on line. The wells-plate or microfluidic chip setup allows monitoring many processes that take part at the gut wall, such as transport, barrier function, metabolic activity, hormone secretion, and the inflammatory response. Special attention will be paid to the possibility to study the impact of your compound on the gut tissue as well as on the interaction of the gut wall with the microbiome.
□ Intestinal explant barrier chip: long-term intestinal absorption screening in a novel microphysiological system using tissue explants.
- 2021.11.12 | Microbiology DMPK
On Tuesday, November 9, 2021 TNO's webinar In vitro microbiome and ex vivo intestinal models entitled "WHAT'S NEW IN IN VITRO MICROBIOME AND EX VIVO INTESTINAL MODELS FOR GUT HEALTH" took place.
You can watch this webinar on demand via this link: